Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine
2021-12-03
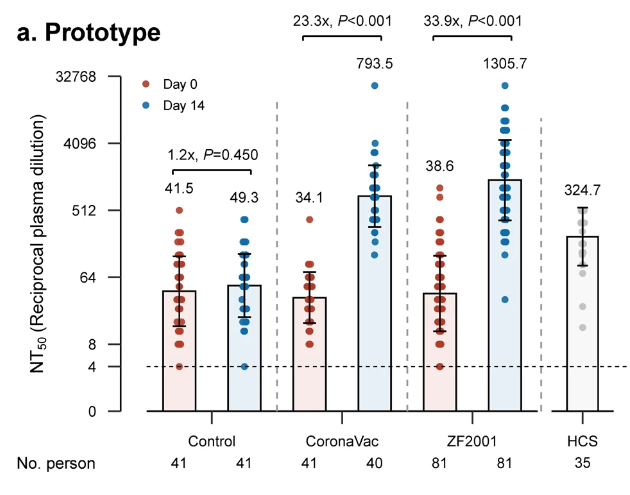
COVID-19 vaccination campaigns are being conducted in countries worldwide, and 47.4% of the world population has received at least one dose of a COVID-19 vaccine.1 Although vaccination has shaped COVID-19 epidemic curves, waning antibody levels and relatively short-duration protection provided by current COVID-19 vaccines have been observed, especially against SARS-CoV-2 variants of concern (VOCs) and among older individuals.2 Booster dose programs have been started in nearly 50 countries, and preliminary evaluation shows that the additional doses reduce breakthrough infections and numbers of symptomatic cases.1,3 The World Health Organization now recommends that for Sinovac and Sinopharm inactivated vaccines, immunization programs should offer an additional (third) dose of the homologous vaccine for people 60 years and older as part of an extended primary series, and that heterologous platforms vaccine for the additional dose may also be considered based on vaccine supply and access considerations. Interim results from heterologous prime-boost studies showed that boosting with heterologous platform vaccines could induce significantly higher titers of neutralizing antibodies and better cellular immunity, providing evidence for programmatic consideration of an alternative to homologous boosting.